Therapeutic Oligos
Custom Oligo Synthesis Coupled With High-Throughput In-Vitro Screening.
Overview
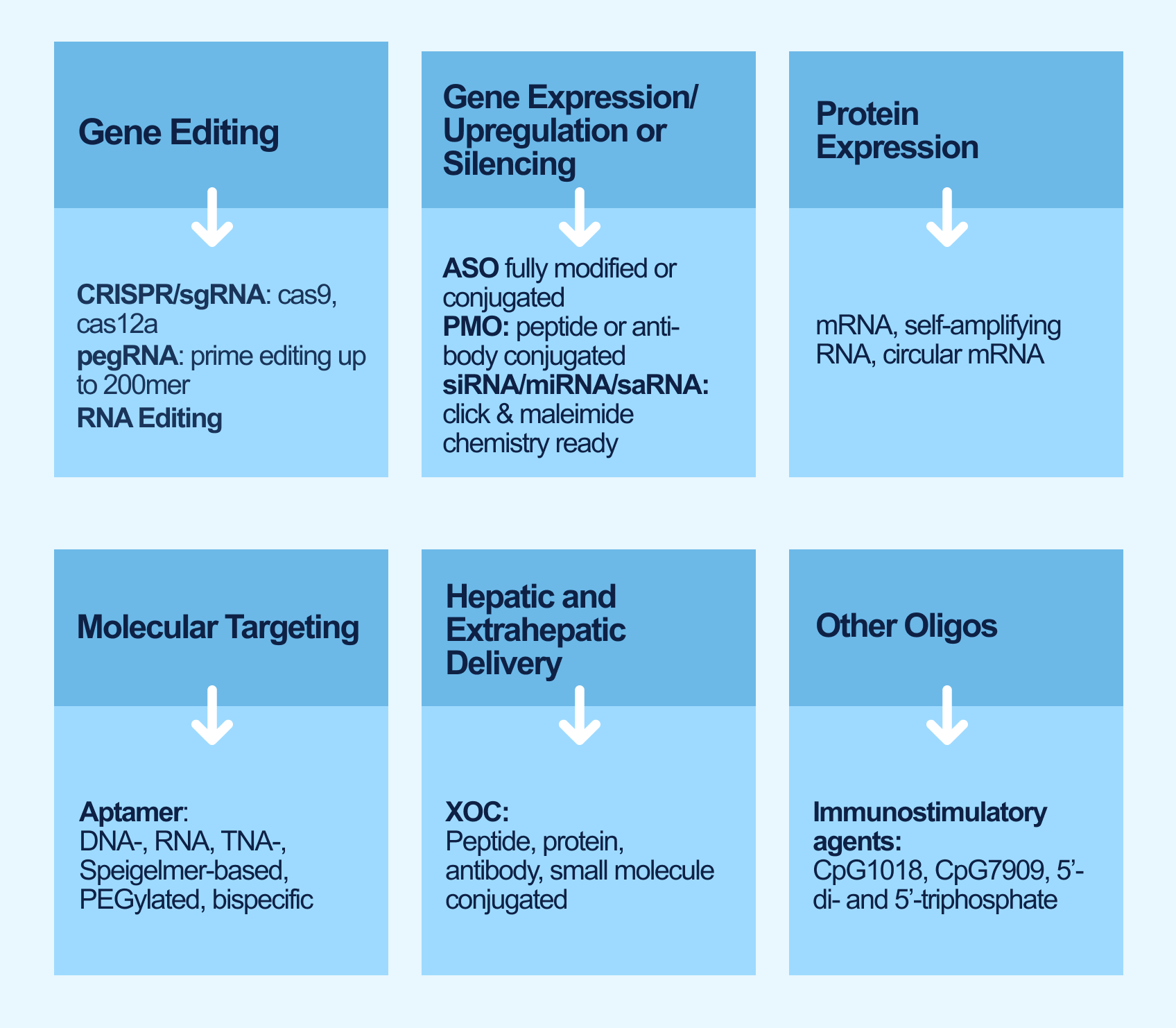
With the rise of targeted therapies powered by antisense, siRNA, and CRISPR technologies, oligonucleotides have become essential components in advancing modern medicine. At Synoligo, we specialize in delivering high-quality, custom oligos designed to meet the unique needs of clients across these cutting-edge therapeutic fields. From early-stage concepts to preclinical development, our team is committed to supporting the evolving demands of the biotech industry with precision, reliability, and deep expertise.
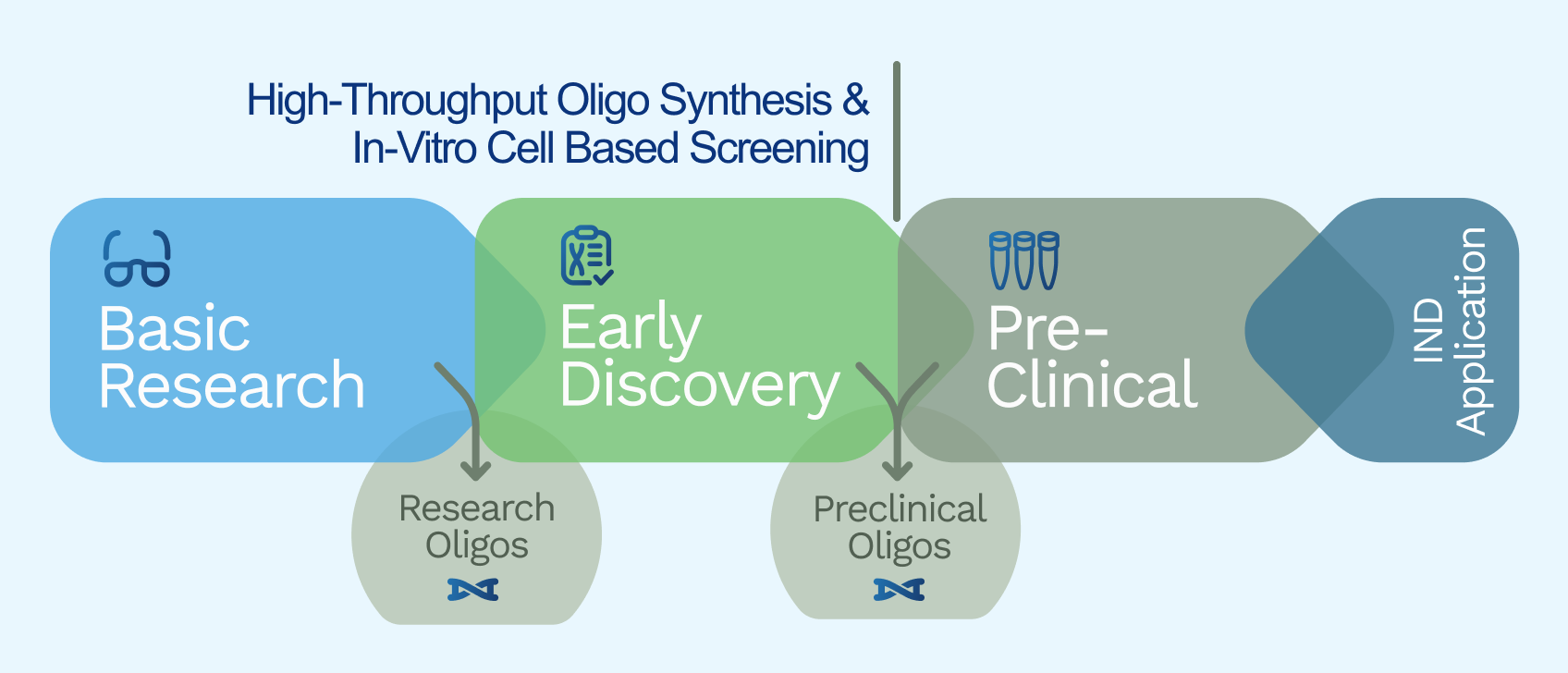
Stages We Cover
Explore our comprehensive solutions tailored for the development, manufacturing and testing of oligonucleotides from research to preclinical stage.
Find Your Lead Candidates Faster With Us!

Overview
With our robust and streamlined workflows, we can rapidly synthesize and screen oligonucleotide libraries to identify leads with optimal activity, minimal toxicity, and strong target engagement, enabling faster, data-driven development decisions.
Why a One-Stop Shop Matters:
End-to-End Support
Fewer Handoffs for a
Seamless Process
Quick Data for Go/No-Go
Decisions
Fastest Path to Lead
Optimization
High-Throughput & Scalable Synthesis of Modified Oligos
Cell-Based Screening for Potency and Selectivity
In-Vitro Assessment of Cell-Based Toxicity and Immune Response
High-Throughput & Scalable Synthesis of Modified Oligos
-
Deliver large quantities of modified oligos within 3 weeks:
- Up to 5,000 modified oligos at 1 nmol scale.
- Up to 200 modified oligos for mouse studies.
- Up to 100 modified oligos for non-GLP NHP studies.
- Delivered in 96 or 384-well plates at your desired concentration for cell screening.
Advanced Modification Expertise (see our full mod list)
- Rare and common 2′-position ribosugar modifications.
- Various backbone phosphoryl-linkages.
Custom Conjugation
- On-resin or post-synthesis conjugation of lipids, carbohydrates, peptides, and more.
Cell-Based Screening for Potency and Selectivity
- Rapidly screen > 240 oligos/week in a wide range of cell models by qRT-PCR.
- Customized luciferase reporter assay for ON/OFF-target screening.
- Provide high-throughput nucleic acid extraction and purification ready for sequencing and downstream selectivity analysis.
In-Vitro Assessment of Toxicity and Immune Response
-
Ability to measure cell death and toxicity using:
- Cell titer/glo.
- LDH release assays.
- Caspase 3/7 assays.
- Screening with specific pathway dependent reporter cell models.
- Screening for cytokine response in primary human PBMCs.