If you go to https://clinicaltrials.gov/ and search “oligonucleotide” as the treatment, you’ll come up with over 260 trials which covers a variety of types of therapeutics modalities – ASOs, siRNAs, sgRNAs, and aptamers-most of which contain phosphorothioate linkages in the backbone.
The reason for this is two-fold. First, the phosphorothioate linkages (PS) substantially increase nuclease resistance. Second, they increase protein binding, predominantly to albumin, which increases tissue distribution and the oligo half-life.

As a result, most therapeutic oligos in clinical studies have one or more PS linkages. However, an added benefit of having PS linkages in the backbone is that it provides a facile means of labeling the therapeutic oligo with a stable isotopic label.
For an internal standard to yield accurate results, it should have the same tissue distribution after administration as the API (active pharmaceutical ingredient) and have essentially the same chemical properties – e.g. charge, hydrophobicity, overall size and conformation. Even subtle differences can have a large impact in terms of extraction efficiency from tissue homogenates, HPLC retention times and ionization efficiency – which is critical for accurate analysis by mass spectrometry. Stable Isotopic Labels (SILs) are essentially the perfect internal standard for APIs. Their chemical properties are essentially indistinguishable from the API because they only differ in the number of neutrons in the nucleus of a particular element and have none of the safety issues of radioactive isotopes. As with all things, however, there are exceptions. If a stable isotope substantially increases the mass of the molecule compared to the isotope with the highest natural abundance, the heavier isotope can change the molecule’s properties. The best example of this is D2O vs H2O. Using deuterated water increases its molecular weight by 11%. As a result, the melting point increases from 0 °C to 3.8 °C and similarly, the boiling point increases from 100 °C to 101.4 °C.
The stable isotope of sulfur, 34S, has essentially no impact upon the properties of the PS-modified oligo other than its overall mass, increasing the molecular weight of a fully-P34S substituted oligo by approximately 0.6%. However, that small increase in mass allows it to be used as an excellent internal standard for pharmacokinetic (PK) and pharmaco-biodistribution (PD) studies by mass spectrometry with even a limited number of 34S PS linkages. In addition, incorporating the 34S PS linkage is compatible with standard automated DNA/RNA synthesis by using a 34S-modified sulfurizing reagent such as 34S-PADS (phenylacetyl disulfide), making it a cost-effective modification. To verify the utility of 34S PS-labeled ASOs as a Stable Isotopic Labels, Stulz et al., [1] prepared two ASO sequences shown in Fig. 1 where * (in black) indicates a standard PS linkage and * (in red) indicates the 34S-SIL PS linkage synthesized using 34S-PADS during sulfurization.

The 34S-ASO was used to generate calibration curves from both liver and kidney homogenates by serial dilution of the labeled and unlabeled ASO stock solutions followed by phenol-chloroform extraction. To verify the accuracy of the calibration curves in an actual animal model, the ASO (at 1mg per injection) was administered to two groups of Beagle dogs by subcutaneous injection. The ASO was administered to the dogs once daily for 7 or 28 days. Afterward, the animals were sacrificed, and liver tissues samples were collected. These calibration curves were then applied to liver samples as shown in Fig. 2. Clearly, using the 34S-SIL ASO internal standard gives much more accurate and consistent results.
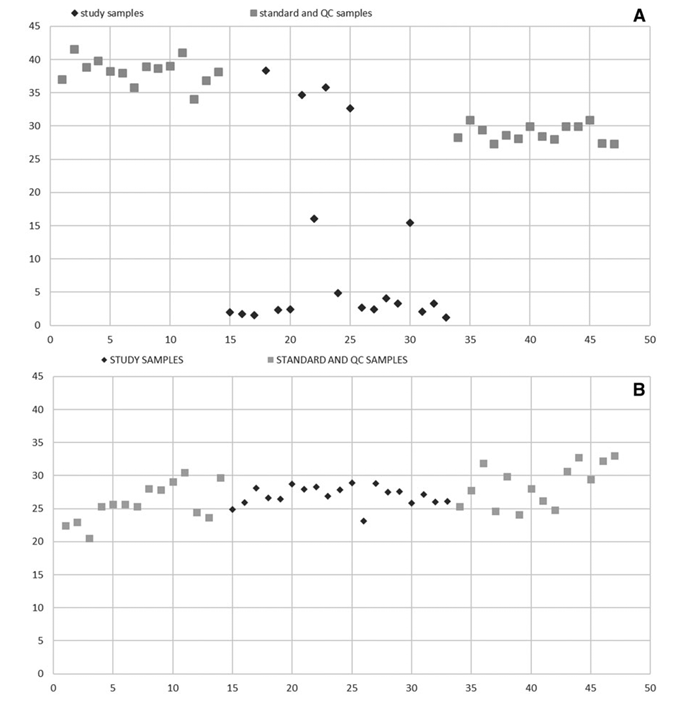
Fig. 2 A comparison of internal standard plots for sample dog liver homogenate extracts compared to standards and QC samples using either (A) an analog ASO internal standard or (B) the 34S-SIL ASO internal standard. Y-axis; Peak area of internal standard for each standard/QC and study sample in batch, X-axis; Injection order of the standards/QCs and study samples in batch. Reprinted from NUCLEIC ACID THERAPEUTICS Volume 31, Number 5, 2021 Mary Ann Liebert, Inc. DOI: 10.1089/nat.2020.0915 under Creative Commons CC BY 4.0 public use license.
In conclusion, most therapeutic oligos contain PS linkages to increase their stability, biodistribution and oligonucleotide half-life. By modifying these phosphorothioate linkages with 34S during the sulfurization step during oligonucleotide synthesis, quantitative and robust internal standards for their corresponding therapeutic oligo are obtained. With identical retention times, physical properties and ionization potential, corrections for matrix effects and extraction recovery can be accurately applied for use in PK and PD studies.
References
[1] 34S-SIL of PCSK9-Active Oligonucleotide as Tools for Accurate Quantification by Mass Spectrometry. Stulz et al., Nucleic Acid Therapeutics Volume 31, Number 5, 2021 DOI: 10.1089/nat.2020.0915
