What backbone chemistries have been used for stereopure oligonucleotides?
Traditional oligonucleotide synthesis using standard phosphoramidite methods does not control stereochemistry, resulting in a stereorandom phosphorothioate (PS) mixture where each linkage may adopt either R or S configuration. Studies in retinal disorders, CNS diseases, and muscular atrophy have demonstrated that stereopure oligonucleotides can significantly impact biological activity in antisense oligos and other therapeutic modalities [1,2,4].
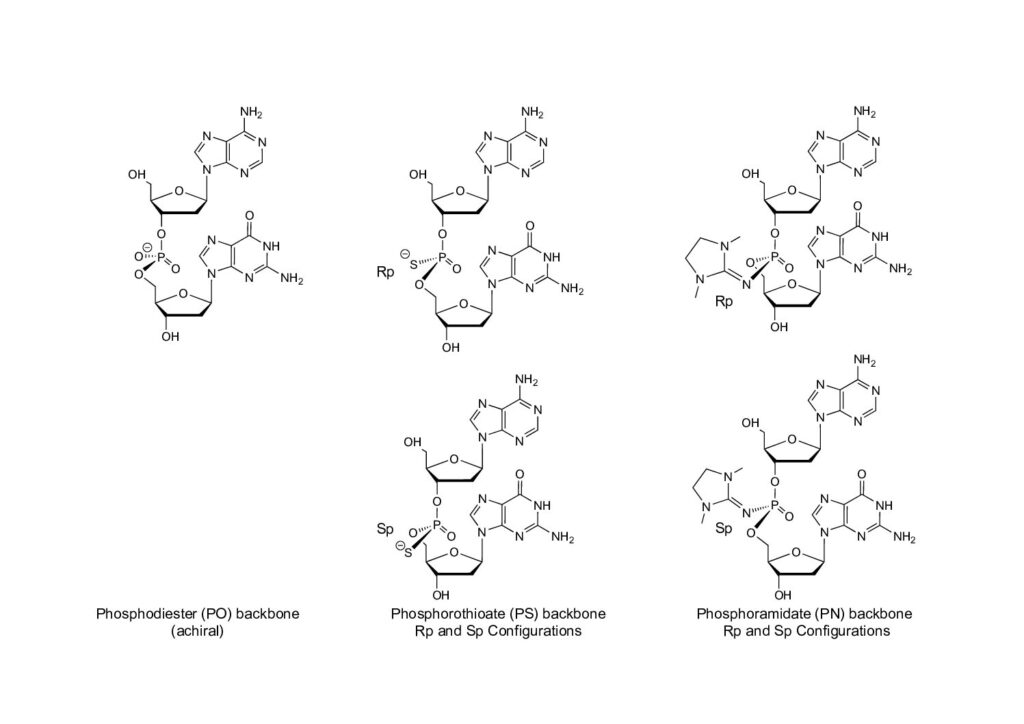
Recognizing the importance of stereochemistry, Wave Life Sciences has been pioneering new developments, including PN (phosphoroamidate) backbone chemistry (See Image 1). PN chemistry has shown promise in enhancing potency, exposure, and durability in therapeutic applications [3].
The phosphorothioate (PS) backbone, commonly used to improve metabolic stability and cellular uptake, plays a vital role in therapeutic oligos [1]. However, its use can sometimes trigger immune responses. To address these challenges, researchers are exploring hybrid backbone strategies that combine PS with other backbones, such as phosphodiester (PO) or phosphoroamidate (PN), to fine-tune oligonucleotide performance and therapeutic efficacy.
Which modalities have been explored with stereochemistry modification?
Stereochemistry modifications have shown potential to enhance therapeutic effects and have been studied across several modalities, including ASOs, siRNA, and splice-switching oligonucleotides.
Antisense Oligonucleotides (ASOs):
A study in 2022 compared the effects of three ASOs targeting Huntington’s disease, focusing on different stereochemistry approaches. Tominersen, developed by Ionis and Roche, uses a stereorandom approach and pan-silencing mechanism to reduce both mutant and wild-type HTT levels. While it demonstrated an ability to lower mHTT in cerebrospinal fluid, higher doses worsened clinical outcomes for some groups, leading to its suspension of Phase 3 trials. Wave Life Sciences pursued stereopure design with its allele-selective antisense oligonucleotides (ASOs), WVE-120101 and WVE-120102, aimed at selectively reducing mHTT. However, clinical trials (PRECISION-HD1 and PRECISION-HD2) demonstrated no significant reduction in mHTT levels compared to placebo. As a result, Wave discontinued the development of these ASOs in 2021 and started developing a new ASO with PN backbone [2]. These results highlight the challenges of optimizing ASO design with stereochemistry for therapeutic benefit.
Gene Silencing:
Wave Life Sciences studied stereopure siRNAs incorporating both PS and phosphoryl guanidine-containing PN backbones. In their experiments with GalNAc-conjugated siRNAs, this combination significantly improved the potency and durability of mRNA silencing in mouse hepatocytes [3].
Splice Switching:
Recent studies on exon-skipping oligonucleotides, used for splicing modulation, demonstrated that chimeric stereopure oligos with both PS and PN backbones enhanced efficacy and pharmacology. In a dystrophic mouse model, these oligos restored sufficient dystrophin levels to improve muscle function [4].
These findings highlight the importance of balancing backbone chemistries and modifications to optimize performance across modalities. However, identifying the right candidate through screening can be resource-intensive. Our team of experts can simplify this process by producing high-throughput oligos, including sgRNA, siRNA, and ASOs, with customizable stereochemistry modifications. With our rapid turnaround times, we help drive your projects forward, delivering results without delay.
How do stereopure oligonucleotides get synthesized?
Synoligo employs industry standard solid-phase synthesis for producing all oligonucleotides, including stereopure oligos. This method offers several key advantages: (1) precise stereocontrol at each phosphorothioate (PS) linkage; (2) the ability to wash and filter the solid support to remove byproducts; and (3) high scalability and compatibility with automated synthesizers.
In solid-phase synthesis, nucleoside oxaphospholidine monomers are commonly used to create stereopure oligos. Wave Life Sciences developed the PRISM platform to produce stereopure oligonucleotides for their therapeutic programs. Their process involves an iterative sulfurization step between capping and detritylation to achieve stereopure PS linkages. For phosphoryl guanidine (PN) linkages, a modified amidite cycle is applied [5,6]. The synthesis continues with cleavage from the support, deprotection, purification, and quality control to ensure a high-quality product.
At Synoligo, we specialize in producing stereopure oligos with phosphoryl guanidine incorporated into PN backbones. Our expertise also extends to substituting phosphoryl guanidine with other PN chemistries, tailoring the synthesis to your experimental needs. Contact us to discuss how we can support your project!
References:
- Iwamoto, N., Butler, D., Svrzikapa, N. et al. Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides. Nat Biotechnol 35, 845–851 (2017). https://doi.org/10.1038/nbt.3948
- Rook ME, Southwell AL. Antisense Oligonucleotide Therapy: From Design to the Huntington Disease Clinic. BioDrugs. 2022 Mar;36(2):105-119. doi: 10.1007/s40259-022-00519-9. Epub 2022 Mar 7.
- Liu W, Iwamoto N, Marappan S, Luu K, Tripathi S, Purcell-Estabrook E, Shelke JD, Shah H, Lamattina A, Pan Q, Schrand B, Favaloro F, Bedekar M, Chatterjee A, Desai J, Kawamoto T, Lu G, Metterville J, Samaraweera M, Prakasha PS, Yang H, Yin Y, Yu H, Giangrande PH, Byrne M, Kandasamy P, Vargeese C. Impact of stereopure chimeric backbone chemistries on the potency and durability of gene silencing by RNA interference. Nucleic Acids Res. 2023 May 22;51(9):4126-4147. doi: 10.1093/nar/gkad268.
- Kandasamy, P., McClorey, G., Shimizu, M., Kothari, N., Alam, R., Iwamoto, N., Kumarasamy, J., Bommineni, G. R., Bezigian, A., Chivatakarn, O., Butler, D. C. D., Byrne, M., Chwalenia, K., Davies, K. E., Desai, J., Shelke, J. D., Durbin, A. F., Ellerington, R., Edwards, B., Godfrey, J., & Vargeese, C. (2022). Control of backbone chemistry and chirality boost oligonucleotide splice switching activity. Nucleic acids research, 50(10), 5443–5466. https://doi.org/10.1093/nar/gkac018
- Kupryushkin MS, Pyshnyi DV, Stetsenko DA. Phosphoryl guanidines: a new type of nucleic Acid analogues. Acta Naturae. 2014 Oct;6(4):116-8.
- Skvortsova YV, Salina EG, Burakova EA, Bychenko OS, Stetsenko DA, Azhikina TL. A New Antisense Phosphoryl Guanidine Oligo-2′-O-Methylribonucleotide Penetrates Into Intracellular Mycobacteria and Suppresses Target Gene Expression. Front Pharmacol. 2019 Sep 19;10:1049. doi: 10.3389/fphar.2019.01049.
