What are antisense oligonucleotides (ASOs)?
Antisense oligonucleotides (ASOs) are short nucleic acids designed to bind to specific RNA sequences, thereby inhibiting, reducing, restoring, or modifying the expression of target RNA. Over the past two decades, this method of gene silencing at the posttranscriptional stage has become increasingly popular as a therapeutic technology, receiving FDA approval for treating conditions such as muscular and spinal atrophy.
Why is mesyl phosphoramidate being used?
The first generation of modified ASOs aimed to protect the natural phosphodiester bond of the backbone against nuclease digestion. This was achieved by using phosphorothioate oligo-deoxynucleotides (PS-ODNs), where the non-bridging oxygen atom is substituted with a sulfur atom. While this modification increased nuclease resistance and facilitated RNase H recruitment to cleave the RNA strand, PS-modified oligos presented challenges such as insufficient biological activity, off-target toxic effects, and low target engagement.
Recent studies have highlighted a new type of modification, mesyl-phosphoramidate antisense oligonucleotides (ASOs), which was used to target the pro-oncogenic miR-21 in melanoma B16 cells and in a xenograft mouse tumor model [1,2]. The results suggested the modification increases ASOs’ stability in biological environments, facilitates cellular uptake, and enhances intracellular trafficking. With improved pharmacokinetic properties and target engagement, mesyl modification holds promise as a replacement for phosphorothioate (PS) in future antisense oligonucleotide applications, pending further studies.
What is mesyl and how is it synthesized?
Mesyl phosphoramidate oligonucleotides are DNA analogs that replace the natural phosphodiester group with a mesyl (methanesulfonyl) group (known by µ-modification or MsPA).
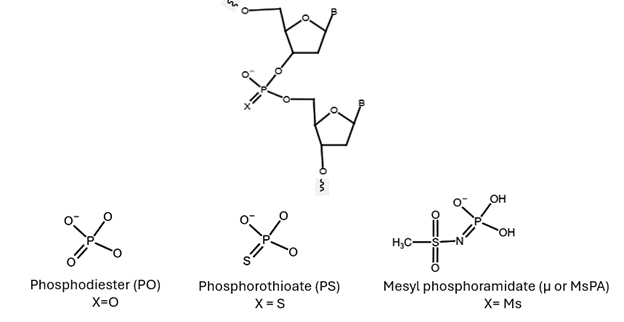
Image 1: Chemical structure of phosphorothioate (PS) and mesyl phosphoramidate that can replace phosphodiester bond (PO)
These oligonucleotides can be synthesized using the traditional solid-phase synthesis method with controlled pore glass or polymer-based support. Synthesis of µ-modification oligos and PS-ODNs follows similar stages but differs in the oxidation step, where an aqueous iodine oxidizer is replaced with 0.5-1 M methanesulfonyl azide in acetonitrile (or other compatible solvents such as ACN or toluene). The Staudinger reaction occurs between the support-bound phosphite triester and mesyl azide within 15-30 minutes, emitting N2. After synthesis, oligonucleotides are cleaved from the support and deprotected using ammonia treatment at 55°C. Purification is carried out using reverse-phase HPLC or conventional gel electrophoresis (PAGE), with purity analysis confirmed by ESI LC-MS or MALDI-TOF MS.
Similarly, close analogs of mesyl phosphoramidate can be synthesized using the same strategy. Caution should be exercised due to the generation of one molecule of nitrogen for every reaction. Avoid recirculating the reaction in a closed-loop system; instead, opt for flow-through mode.
What are the common impurities associated with this modification?
Due to the slow reaction kinetics of the Staudinger reaction, incomplete reactions or hydrolysis can lead to an impurity of -77.1 Da, like PS to PO conversion.
References:
- Miroshnichenko, S. K., Patutina, O. A., Burakova, E. A., Chelobanov, B. P., Fokina, A. A., Vlassov, V. V., Altman, S., Zenkova, M. A., & Stetsenko, D. A. (2019). Mesyl phosphoramidate antisense oligonucleotides as an alternative to phosphorothioates with improved biochemical and biological properties. Proceedings of the National Academy of Sciences, 116(4), 1229–1234. https://doi.org/10.1073/pnas.1813376116
- Patutina, O. A., Gaponova Miroshnichenko, S. K., Sen’kova, A. V., Savin, I. A., Gladkikh, D. V., Burakova, E. A., Fokina, A. A., Maslov, M. A., Shmendel’, E. V., Wood, M. J. A., Vlassov, V. V., Altman, S., Stetsenko, D. A., & Zenkova, M. A. (2020). Mesyl phosphoramidate backbone modified antisense oligonucleotides targeting miR-21 with enhanced in vivo therapeutic potency. Proceedings of the National Academy of Sciences of the United States of America, 117(51), 32370–32379. https://doi.org/10.1073/pnas.2016158117