In a recent Blog Post entitled, Using Stable Isotope Labeling with 34S for Pharmacokinetic and Biodistribution Studies, the advantages of using stable isotopic labels (SIL) to generate an internal standard was discussed.
Summary of SIL:
- Using a SIL of the active pharmaceutical ingredient (API) generates an internal standard whose chemical and physical properties are essentially identical to the API, allowing for quantitative and accurate pharmacokinetic and biodistribution measurements with the use of High-Resolution Mass Spectrometry.
- SIL internal standards have none of the safety nor regulatory issues that are associated with their radioactive counterparts.
- Using 34S-labeled sulfurizing reagents during solid phase oligonucleotide synthesis provides a convenient and cost-effective means of adding the SIL.
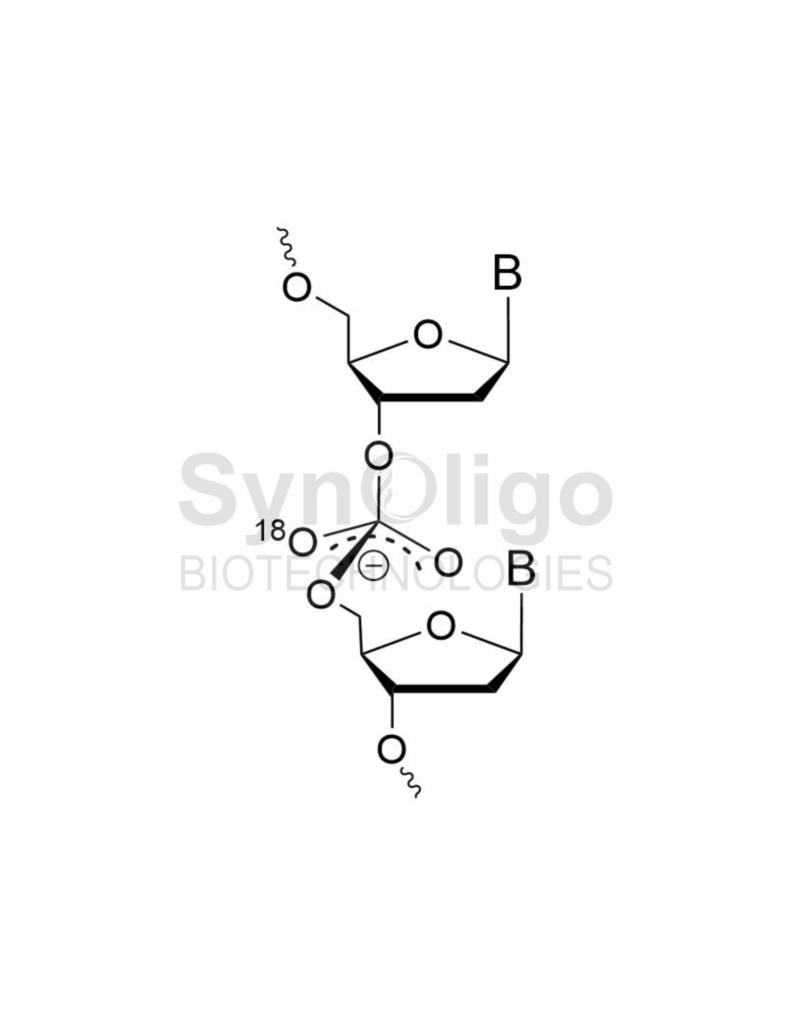
We would be remiss not to also mention another isotope amenable to labeling oligonucleotides – 18O. Like 34S, the isotopic label is added during the oxidation step of the phosphite; however, in this case it is to generate a standard phosphodiester (PO) linkage rather than the nuclease resistant phosphorothioate (PS). Some classes of therapeutic oligos have predominantly natural phosphodiester backbones – siRNAs and sgRNAs – making them excellent candidates for 18O SIL internal standards. The simplest way of incorporating 18O into the phosphodiester backbone site-specifically, is to prepare an Iodine Oxidation solution using THF, pyridine, and 18O water as described by Ohgi, et al.,[1] who obtained excellent results.
We are happy to offer 18O- and 34S-labeled oligonucleotides to our growing list of modifications available from Synoligo.
References
[1] Synthesis of 18O-labeled RNA for application to kinetic studies and imaging, Ohgi, et al., Nucleic Acids Research Vol 41(12) e126, 2013 https://doi.org/10.1093/nar/gkt344
