Monoclonal antibodies provide exquisite targeting specificity for different cell types, markers and cell-surface receptors. When conjugated to therapeutic oligos, such as siRNAs and antisense oligos, the antibody facilitates the internalization of the therapeutic oligo by receptor-mediated endocytosis. In a previous article Choosing Linkers for Antibody-Oligonucleotide Conjugates (AOCs) versus for Antibody-Drug Conjugates (ADCs), we focused on the advantages and disadvantages of the different classes of linkers and briefly described their regioselective labeling of antibodies in the hinge region. This article will focus on different strategies to achieve site-specific labeling of native antibodies by enzymatic means.
Background
There are five isotypes of immunoglobulins—IgA, IgD, IgE, IgG, and IgM. With the exception of IgM, they all share the same basic structure: two identical heavy chains and two identical light chains which are crosslinked by disulfide bonds forming a symmetric Y-shaped structure. (IgM is unique in that it is a pentameric structure of five Y-shaped subunits which are crosslinked). The ‘head’ of the antibody is known as the Fab (for Antigen binding fragment) and below the hinge is the Fc region (crystallizable fragment) – their names coined by Rodney Porter who treated antibodies with papain which caused cleavage at the hinge region. He found one fraction crystallized over time while the other, disordered protein fraction bound the antigen. He shared a Nobel prize for determining the structure of antibodies with Gerald Edelmann in 1972. The Fab terminates with six hypervariable loops known as Complementarity-determining Regions (CDRs) with three on the heavy chain CDR loops and three light chain CDR loops on each arm of the antibody. The CDR loops are responsible for binding antigens.[1]
IgG and IgA are further divided into subclasses: IgG1, IgG2, IgG3, IgG4, and IgA1 and IgA2. All are N-glycosylated on specific asparagine (Asn) residues on the crystallizable fragments (Fc) of the heavy chains. Shown in Fig. 1 is a ribbon representation of IgG from the Protein Data Bank for murine IgG2a (unlike humans, mice have two subtypes of IgG2, a and b). The heavy chains are shown in blue and cyan, and the light chains are shown in green. Three disulfide bonds in the hinge region are shown in yellow. The glycan (brown) occupies the same location in both mice and humans at Asn297. Both the light and heavy chains contain constant and variable regions. The IgG heavy chain contains three constant regions—CH1, CH2, and CH3—whereas the light chain contains a single constant region, CL. Both chains contain a single variable region, VL and VH.
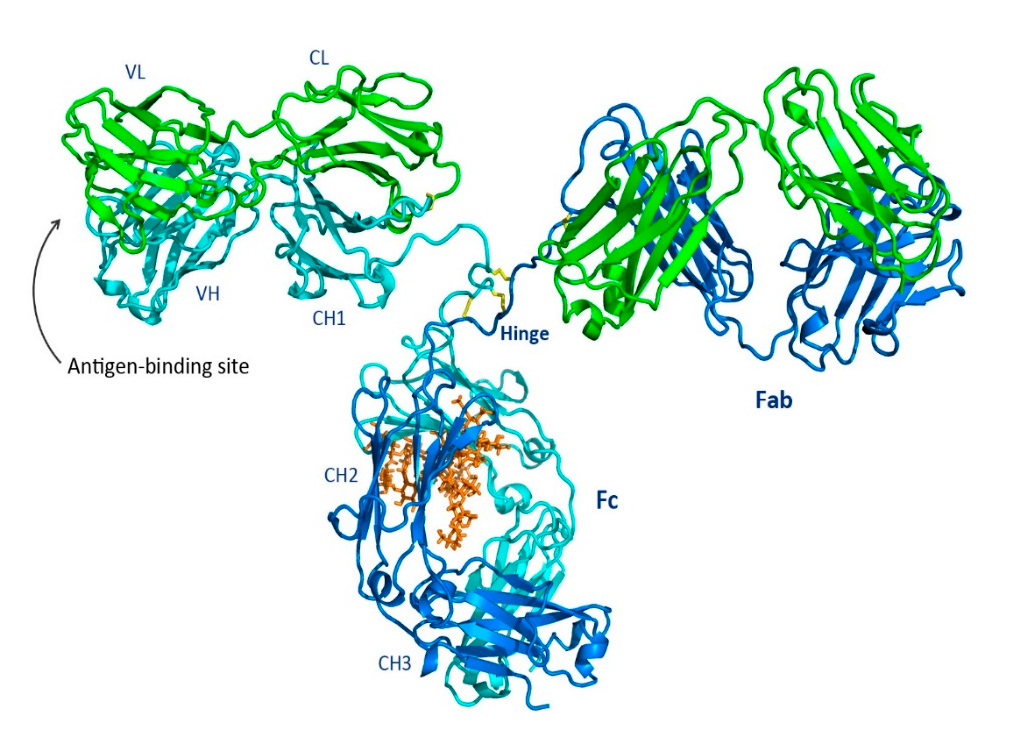
Fig. 1 Shown is a ribbon representation of IgG from the Protein Data Bank of the murine IgG2a. The heavy chains are blue and cyan, and the light chains are shown in green. Three disulfide bonds in the hinge region are shown in yellow. The glycan (brown) is located at the same position in both mice and humans at Asn297. Reprinted from Chiu, Mark; Goulet, Dennis; Teplyakov, Alexey; and Gilliland, Gary. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies, vol. 8, p. 55 (2019) under the Creative Commons CC BY 4.0 public use license.
It is the Fc region that serves as the effector of the antibody, where binding to IgG-specific receptors, such as Fc gamma receptors (FcγR), occurs. The Fc region is also the site where C1q binds, initiating activation of the complement system and leading to antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP).[1] A variety of Fc gamma receptors exist, as shown in Table 1, and are expressed predominantly on leukocytes involved in both the innate and adaptive immune responses, including macrophages, neutrophils, natural killer cells, and T and B lymphocytes.[1],[2] The extent of binding to Fc gamma receptors can be modulated and even abolished by changes in the glycan at Asn297. Asn297 is conserved across all IgG isotypes and, in fact, is conserved in essentially all known mammals, including camelids. IgG3 contains a second conserved asparagine residue, Asn392, which is only weakly glycosylated in approximately 12% of IgG3 antibodies.[3]
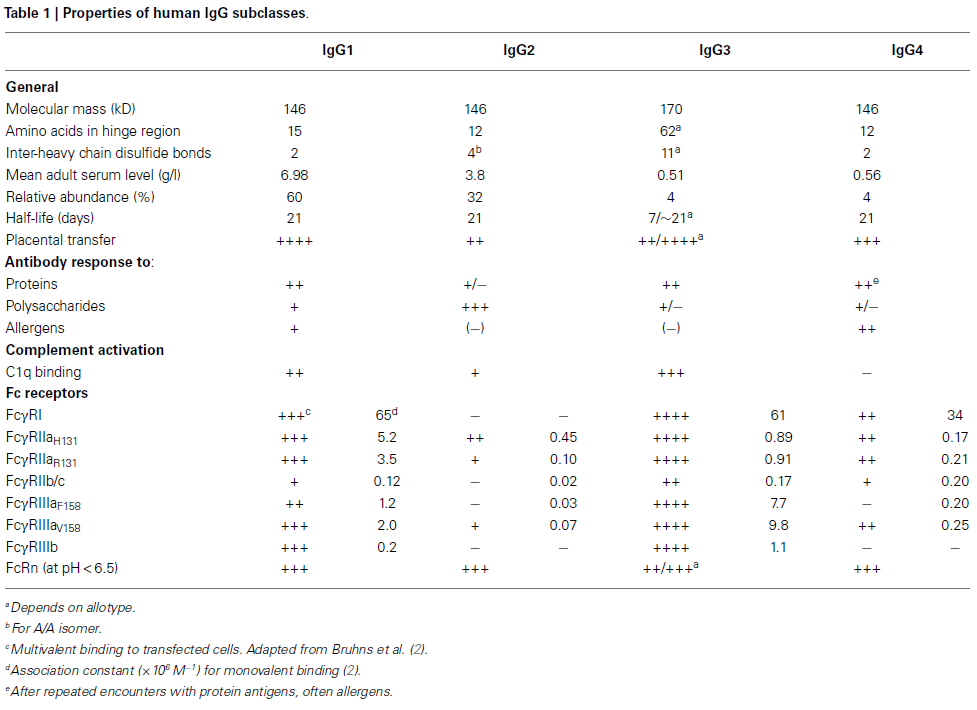
The glycan attached to Asn297 is a biantennary complex. Shown in Fig. 2 are the constituent sugars. Of particular interest are fucose and sialic acid (shown as red triangles and fuchsia diamonds, respectively, in Fig. 2). When the glycan is fucosylated, binding to the FcγRIIa is significantly reduced, leading to a decrease in antibody-dependent cellular cytotoxicity (ADCC). Sialylation, on the other hand, appears to increase binding to the FcγRIIb, an anti-inflammatory receptor, thereby reducing the immunogenic response of IgG bearing sialic acid on the glycan antenna.[4] Interestingly, the addition of a bisecting N-acetylglucosamine (GlcNAc) does not affect binding to the FcγRIIa but instead sterically prevents fucosylation, resulting in increased ADCC.[5]
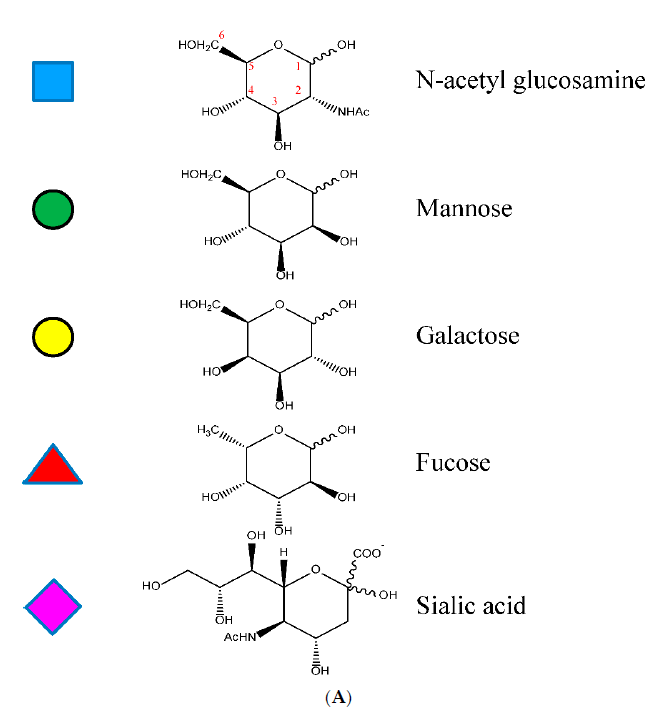
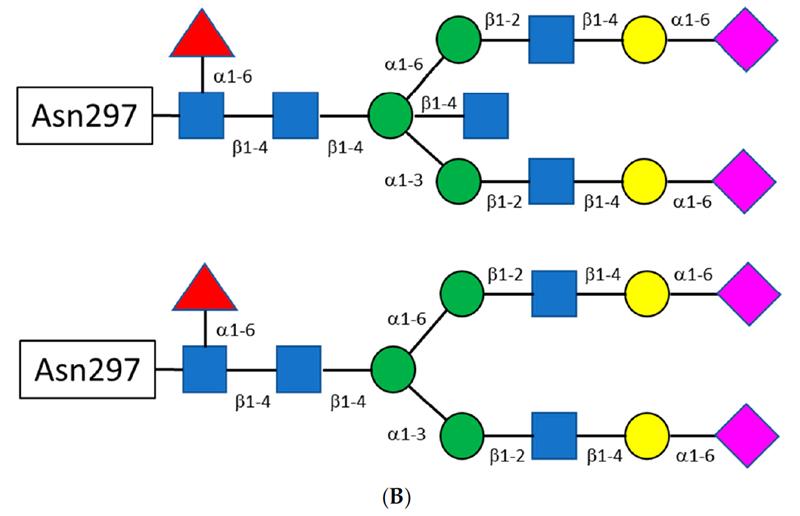
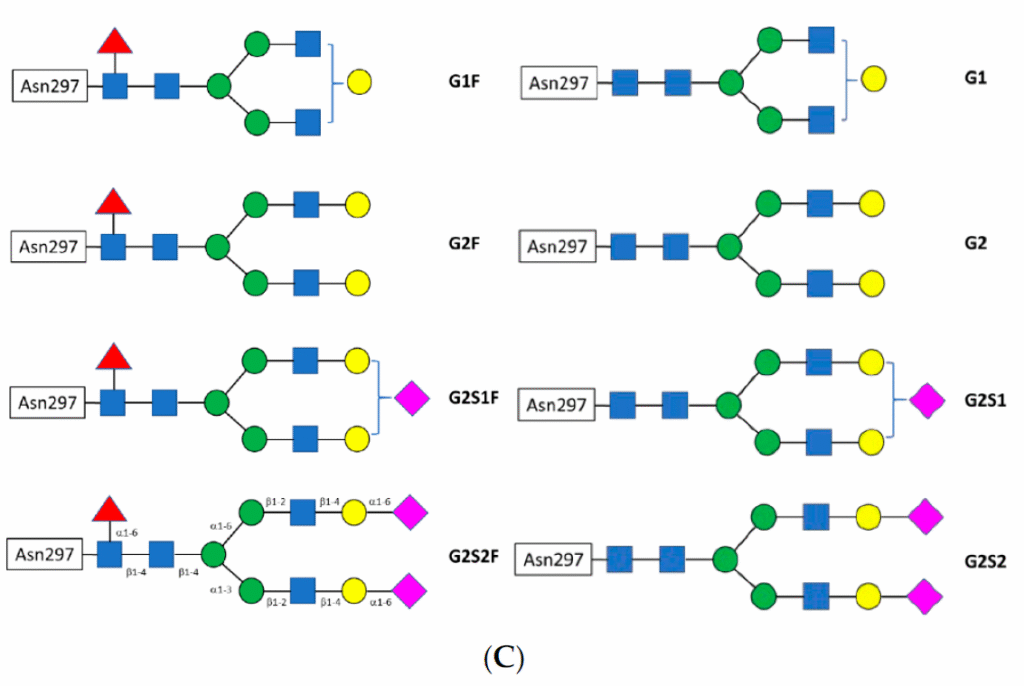
Fig. 2 A) Shown are the structures of the constituent sugars typically found in the biantennary glycans off Asn297. B) A schematic for the biantennary glycans showing the glycosidic bonds between the sugars. The figure on top shows a bisecting N-acetyl glucosamine off the central mannose. C) Examples of the variation of the major glycans observed. Reprinted from Chiu, Mark & Goulet, Dennis & Teplyakov, Alexey & Gilliland, Gary. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies, vol 8, p55 (2019) under Creative Commons CC BY 4.0 public use license.
Site-Specific Enzymatic Labeling of IgGs
Site-specific labeling of antibody-oligo conjugates (AOCs) and antibody-drug conjugates (ADCs) yields a homogeneous Active Pharmaceutical Ingredient (API) with greater ease of purification, characterization and more predictable pharmacokinetics. The first example of site-specific labeling was the THIOMAB platform developed by Genentech which engineered an accessible cysteine for labeling with a maleimide-drug conjugate.[6] However, having to engineer the antibody to have accessible cysteines is time-consuming and in addition, the maleimide-sulfhydryl bond was subject to retro-Michael reaction, leading to cleavage of the drug from the antibody and a smaller therapeutic window due to off-target effects.
A significant improvement was reported by Remon van Geel et al., in which the glycans of native IgG antibodies were remodeled using endoglycosidases (Endo-S or Endo-S2) that cleave the glycan antenna between the first two N-acetylglucosamine residues attached to Asn297.[7] After trimming the glycan to a single fucosylated GlcNAc, a glycosyltransferase, GalT (Y289L), was used to append an azido-modified N-acetyl galactosamine analog. The azide enabled attachment of a cytotoxic payload to the antibody using copper-free click chemistry and a bicyclononyne (BCN) drug conjugate (Fig. 3). The authors noted that removal of the glycan could be advantageous for ADCs due to reduced off-target uptake mediated by binding to FcγR-positive cells. In the absence of an intact glycan, the CH2 region of the Fc is no longer held in an open conformation, resulting in abolished or significantly reduced binding to FcγRs. Indeed, the intact N-glycan of the ADC trastuzumab emtansine appears to be responsible for thrombocytopenia in approximately 10% of treated patients. When antibodies were used to sterically block the CH2 region of the ADC, binding to the FcγRIIa was abolished, preventing off-target uptake by megakaryocytes which had caused the drug-induced thrombocytopenia.[8]
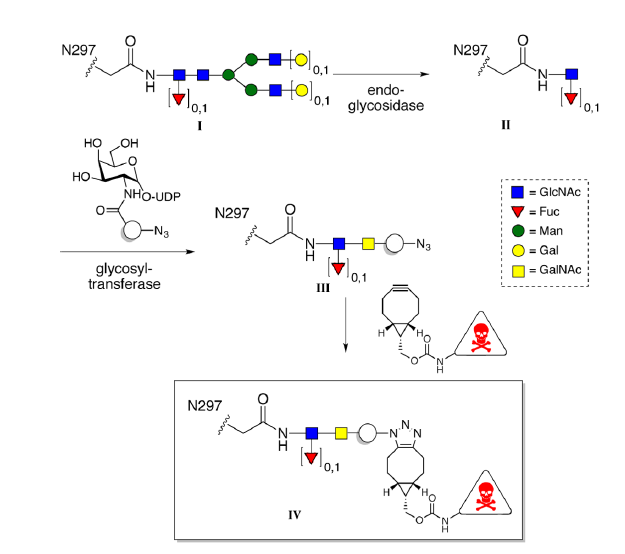
Fig. 3 Shown is the glycan remodeling strategy used to attach a cytotoxic payload to the conserved Asn297 of IgG immunoglobulins. Reprinted from Remon van Geel et al., Chemoenzymatic Conjugation of Toxic Payloads to the Globally Conserved N-Glycan of Native mAbs Provides Homogeneous and Highly Efficacious Antibody–Drug Conjugates, Bioconjugate Chemistry 2015 26 (11), 2233–2242 under the Creative Commons CC BY 4.0 public use license.
While other enzymatic methods exist for site-specific antibody labeling such as GlyConnect™, SMAC™, and ConjuAll™, arguably the most interesting and versatile development emerged from the Wang lab at the University of Maryland. The Wang lab pioneered the use of a mutant Endo-S2 enzyme to remodel glycans on Asn297 using substituted N-acetylglucosamine oxazolines as substrates, even enabling attachment of a complete biantennary sialylated glycan without removing N-glycans present in the Fab region.[9] This approach is advantageous for several reasons. First, N-glycans in the Fab domain are left untouched; approximately one-fifth of all IgG isotypes found in plasma contain N-glycans in the Fab region.[10] Second, during monoclonal antibody production, IgG antibodies expressed in CHO and other mammalian cell types exhibit heterogeneous glycan isoforms that may or may not be fucosylated or sialylated. Such heterogeneity is problematic not only for efficacy and the risk of unwanted immune responses, but also for practical considerations such as smoothly achieving FDA approval. Third, controlled N-glycan remodeling allows tuning of ADC binding to FcγR subtypes, thereby modulating ADCC, CDC, and other immune responses. In addition, the Wang lab demonstrated that EndoS2 can be used to both trim the native IgG N-glycan and attach azide-substituted N-acetylglucosamine disaccharide oxazolines in a single step, even when using drug-preloaded disaccharide oxazolines.[11] Shown in Fig. 4 is a schematic of IgG glycan remodeling using either a disaccharide GlcNAc oxazoline (A) or a complete, sialylated biantennary glycan via EndoS2-D184M.
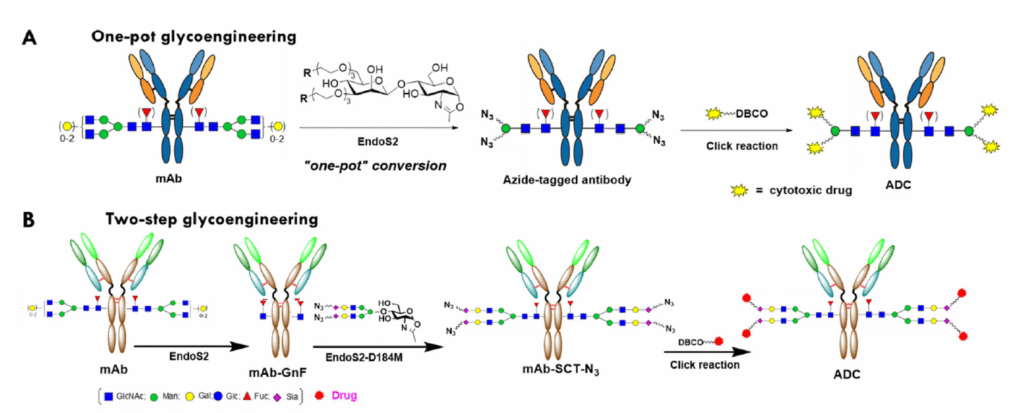
Fig. 4 Schematic of N-glycan remodeling of IgG Asn297 using either a simple disaccharide GlcNAc oxazoline (A) or the complete, sialylated biantennary glycan using EndoS2-D184M (B). Reprinted from Yang, Q. et al., Evaluation of Two Chemoenzymatic Glycan Remodeling Approaches to Generate Site-Specific Antibody–Drug Conjugates. Antibodies 2023, 12, 71, under the Creative Commons CC BY 4.0 public use license.
If you are interested in the site-specific labeling of your antibody, we would be happy to help. Please contact oligosales@synoligo.com
References
[1] Chiu, Mark & Goulet, Dennis & Teplyakov, Alexey & Gilliland, Gary. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies, vol 8, p55 (2019). https://doi.org/10.3390/antib8040055
[2] Vidarsson G, Dekkers G, and Rispens T (2014) IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. 5:520. https://doi.org/10.3389/fimmu.2014.00520
[3] Krištić J, Lauc G. The importance of IgG glycosylation – What did we learn after analyzing over 100,000 individuals. Immunol Rev. 328(1):143-170 (2024). https://doi.org/10.1111/imr.13407
[4] Beneduce C, Nguyen S, Washburn N, Schaeck J, Meccariello R, Holte K, Ortiz D, Manning AM, Bosques CJ, Kurtagic E. Inhibitory Fc-Gamma IIb Receptor Signaling Induced by Multivalent IgG-Fc Is Dependent on Sialylation. Cells. 2023 Aug 23;12(17):2130. doi: https://doi.org/10.3390/cells12172130.
[5]Gerlof P. Bosman, et al., Bisecting N-Acetylglucosamine of the N-Glycan of Immunoglobulin G Does Not Affect Binding to Fc Gamma Receptors,ACS Chemical Biology, 20 (3), 680-689 (2025). DOI: https://doi.org/10.1021/acschembio.4c00807
[6] Junutula JR, Raab H, Clark S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol, 26:925–32 (2008).
[7] Remon van Geel, et al., Chemoenzymatic Conjugation of Toxic Payloads to the Globally Conserved N-Glycan of Native mAbs Provides Homogeneous and Highly Efficacious Antibody–Drug Conjugates, Bioconjugate Chemistry 2015 26 (11), 2233-2242. DOI: https://doi.org/10.1021/acs.bioconjchem.5b00224
[8] Uppal, Hirdesh et al. “Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1).” Clinical cancer research : an official journal of the American Association for Cancer Research 21(1) 123-33 (2015) doi: https://doi.org/10.1158/1078-0432.CCR-14-2093
[9] Wei Huang, John Giddens, Shu-Quan Fan, Christian Toonstra, and Lai-Xi Wang, Chemoenzymatic Glycoengineering of Intact IgG Antibodies for Gain of Functions. J Am Chem Soc. 134(29),12308–12318 (2012). doi: https://doi.org/10.1021/ja3051266.
[10] Krištić J, Lauc G. The importance of IgG glycosylation-What did we learn after analyzing over 100,000 individuals. Immunol Rev. 328(1), 143-170 (2024). doi: https://doi.org/10.1111/imr.13407.
[11] Xiao Zhang, Chong Ou, Huiying Liu, and Lai-Xi Wang, Synthesis and Evaluation of Three Azide-Modified Disaccharide Oxazolines as Enzyme Substrates for Single-Step Fc Glycan-Mediated Antibody-Drug Conjugation, Bioconjugate Chemistry 33 (6), 1179-1191 (2022)DOI: https://doi.org/10.1021/acs.bioconjchem.2c00142
