Check out our updated section below!
What are antisense oligonucleotides (ASOs)?
Antisense oligonucleotides (ASOs) are short nucleic acids designed to bind to specific RNA sequences, thereby inhibiting, reducing, restoring, or modifying the expression of target RNA. Over the past two decades, this method of gene silencing at the posttranscriptional stage has become increasingly popular as a therapeutic technology, receiving FDA approval for treating conditions such as muscular and spinal atrophy.
Why is mesyl phosphoramidate modification being used?
The first generation of modified ASOs aimed to protect the natural phosphodiester bond of the backbone against nuclease digestion. This was achieved by using phosphorothioate oligo-deoxynucleotides (PS-ODNs), where the non-bridging oxygen atom is substituted with a sulfur atom. While this modification increased nuclease resistance and facilitated RNase H recruitment to cleave the RNA strand, PS-modified oligos presented challenges such as insufficient biological activity, off-target toxic effects, and low target engagement.
Recent studies have highlighted a new type of modification, mesyl-phosphoramidate antisense oligonucleotides (ASOs), which was used to target the pro-oncogenic miR-21 in melanoma B16 cells and in a xenograft mouse tumor model [1,2]. The results suggested the modification increases ASOs’ stability in biological environments, facilitates cellular uptake, and enhances intracellular trafficking. With improved pharmacokinetic properties and target engagement, mesyl modification holds promise as a replacement for phosphorothioate (PS) in future antisense oligonucleotide applications, pending further studies.
What is mesyl phosphoramidate and how is it synthesized?
Mesyl phosphoramidate oligonucleotides are DNA analogs that replace the natural phosphodiester group with a mesyl (methanesulfonyl) group (known by µ-modification or MsPA).
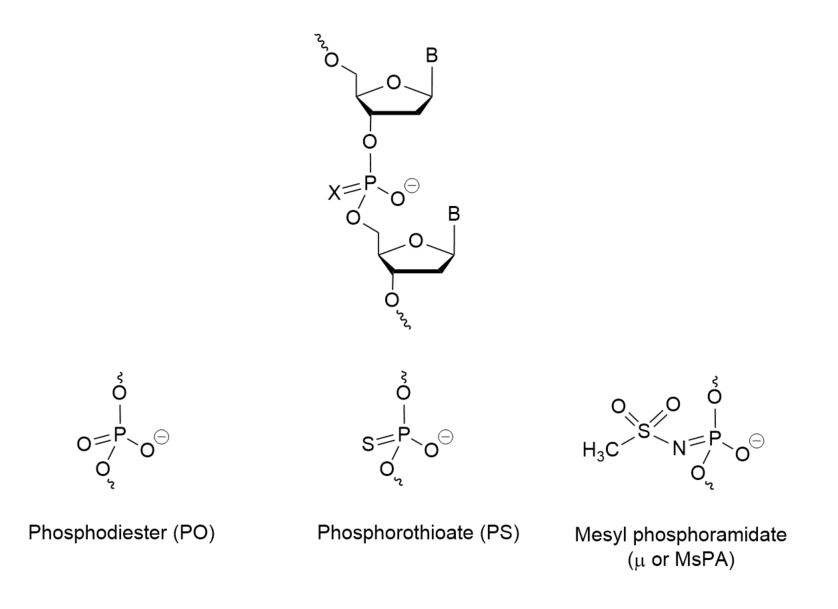
These oligonucleotides can be synthesized using the traditional solid-phase synthesis method with controlled pore glass or polymer-based support. Synthesis of µ-modification oligos and PS-ODNs follows similar stages but differs in the oxidation step, where an aqueous iodine oxidizer is replaced with 0.5-1 M methanesulfonyl azide in acetonitrile (or other compatible solvents such as ACN or toluene). The Staudinger reaction occurs between the support-bound phosphite triester and mesyl azide within 15-30 minutes, emitting N2. After synthesis, oligonucleotides are cleaved from the support and deprotected using ammonia treatment at 55°C. Purification is carried out using reverse-phase HPLC or conventional gel electrophoresis (PAGE), with purity analysis confirmed by ESI LC-MS or MALDI-TOF MS.
Similarly, close analogs of mesyl phosphoramidate can be synthesized using the same strategy. Caution should be exercised due to the generation of one molecule of nitrogen for every reaction. Avoid recirculating the reaction in a closed-loop system; instead, opt for flow-through mode.
What are the common impurities associated with this modification?
Due to the slow reaction kinetics of the Staudinger reaction, incomplete reactions or hydrolysis can lead to an impurity of -77.1 Da, like PS to PO conversion.
New Update:
What other modalities are being explored with mesyl phosphoramidate?
Mesyl phosphoramidate (MsPA) is being actively studied beyond standard antisense oligonucleotides (ASOs), particularly in gapmer ASOs and splice-switching oligos. Researchers are also exploring combinations of MsPA with other backbones and sugar modifications to maximize therapeutic potential.
Gapmer ASOs:
1. Mesyl phosphoramidate (MsPA) + 2’-O-methyl (2’-OMe):
Ionis Pharmaceuticals demonstrated that combining MsPA with 2’-OMe can enhance the activity of gapmer PS-ASOs. Replacing PS linkages with MsPA at the 5’ or 3’ wings of the gap increased RNAse H1 cleavage rates. Moreover, the inclusion of 2’-OMe at position 2 of the gap can significantly reduced cytotoxicity [3].
2. Stereochemistry (PS + MsPA):
Research into the stereochemistry of MsPA within gapmer ASOs revealed that while MsPA chirality does not significantly impact antisense activity, the positional placement does improve resistance to 3’-5’ exonuclease digestion, a critical factor for stability and efficacy [4].
3. MsPA + Busyl phosphoramidate + PSMA ligand:
A recent study combined mesyl and busyl phosphoramidate backbones with PSMA ligands to enhance delivery and activity against Malat1 lncRNA in prostate cancer cells. This partially modified ASO outperformed all-PS ASOs in efficient Malat1 lncRNA depletion in vitro. [5].
Splice Switching:
Hammon et al. investigated MsPA and busyl phosphoramidates in splice-switching oligos designed for exon inclusion in spinal muscular atrophy (SMA) fibroblasts. These backbones, paired with 2’-OMe and 2’-MOE sugars, showed no significant difference in splice switching activity to their controls. Highlighted challenges, such as inefficient endosomal release and nuclear uptake, suggest the need for optimization and exploration of cellular delivery pathways [6].
These studies highlights the versatility of MsPA and its potential in various oligonucleotide applications. While not every combination yields optimal results, these efforts pave the way for new oligonucleotide designs.
Whether for screening, lead optimization, or advanced therapeutic research, Synoligo is ready to synthesize oligos with the modifications you need. Let’s collaborate to advance your project!
References:
- Miroshnichenko, S. K., Patutina, O. A., Burakova, E. A., Chelobanov, B. P., Fokina, A. A., Vlassov, V. V., Altman, S., Zenkova, M. A., & Stetsenko, D. A. (2019). Mesyl phosphoramidate antisense oligonucleotides as an alternative to phosphorothioates with improved biochemical and biological properties. Proceedings of the National Academy of Sciences, 116(4), 1229–1234. https://doi.org/10.1073/pnas.1813376116
- Patutina, O. A., Gaponova Miroshnichenko, S. K., Sen’kova, A. V., Savin, I. A., Gladkikh, D. V., Burakova, E. A., Fokina, A. A., Maslov, M. A., Shmendel’, E. V., Wood, M. J. A., Vlassov, V. V., Altman, S., Stetsenko, D. A., & Zenkova, M. A. (2020). Mesyl phosphoramidate backbone modified antisense oligonucleotides targeting miR-21 with enhanced in vivo therapeutic potency. Proceedings of the National Academy of Sciences of the United States of America, 117(51), 32370–32379. https://doi.org/10.1073/pnas.2016158117
- Zhang L, Liang XH, De Hoyos CL, Migawa M, Nichols JG, Freestone G, Tian J, Seth PP, Crooke ST. The Combination of Mesyl-Phosphoramidate Inter-Nucleotide Linkages and 2′-O-Methyl in Selected Positions in the Antisense Oligonucleotide Enhances the Performance of RNaseH1 Active PS-ASOs. Nucleic Acid Ther. 2022 Oct;32(5):401-411. doi: 10.1089/nat.2022.0005. Epub 2022 Jul 20.
- Anderson BA, Freestone GC, Low A, De-Hoyos CL, Iii WJD, Østergaard ME, Migawa MT, Fazio M, Wan WB, Berdeja A, Scandalis E, Burel SA, Vickers TA, Crooke ST, Swayze EE, Liang X, Seth PP. Towards next generation antisense oligonucleotides: mesylphosphoramidate modification improves therapeutic index and duration of effect of gapmer antisense oligonucleotides. Nucleic Acids Res. 2021 Sep 20;49(16):9026-9041. doi: 10.1093/nar/gkab718.
- Sergeeva, O., Akhmetova, E., Dukova, S., Beloglazkina, E., Uspenskaya, A., Machulkin, A., Stetsenko, D., & Zatsepin, T. (2024). Structure-activity relationship study of mesyl and busyl phosphoramidate antisense oligonucleotides for unaided and PSMA-mediated uptake into prostate cancer cells. Frontiers in chemistry, 12, 1342178. https://doi.org/10.3389/fchem.2024.1342178
- Hammond, S. M., Sergeeva, O. V., Melnikov, P. A., Goli, L., Stoodley, J., Zatsepin, T. S., & Wood, M. J. (2021). Mesyl phosphoramidate oligonucleotides as potential splice-switching agents: impact of backbone structure on activity and intracellular localization. nucleic acid therapeutics, 31(3), 190-200.
