What are 5′ triphosphate oligonucleotides and how are they being used?
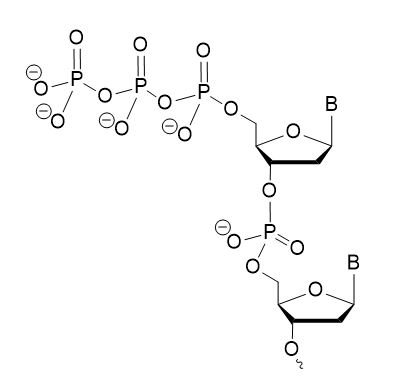
5′ triphosphate (5′-PPP) oligonucleotides are short strands of nucleic acids with a triphosphate group attached to their 5′ end (Image 1). This 5′-triphosphate plays crucial roles in biological processes, particularly in RNA biology. Incorporating 5′-PPP into RNA or DNA oligos enables a variety of biological and biomedical applications.
For 5′-triphosphate RNA oligos:
1. Immunotherapy and vaccine adjuvants, stimulating antiviral defense.
2. Gene silencing, where 5′-PPP-modified siRNA silences specific genes.
3. CRISPR applications, using 5′-PPP-modified guide RNAs to activate immune pathways or serve as biomarkers in gene editing.
Additionally, modifying the 5′-PPP cap on RNA oligos protects them from degradation, enhancing processing and translation [1].
For 5′-triphosphate DNA oligos:
1. They can function as decoy oligos to inhibit DNA-binding proteins.
2. Act as substrates for enzymatic reactions.
3. Support viral DNA replication studies.
What synthesis method is used for 5′ triphosphate oligonucleotide?
The enzymatic synthesis of 5′-triphosphate oligonucleotides is a common method, but it has limitations based on sequence, size, and enzyme bias toward specific nucleotides [2]. A more favorable approach is the chemical method, which utilizes solid-phase phosphoramidite synthesis. In this process, nucleotides are sequentially added from the 3′ to the 5′ direction, leaving a free 5′ hydroxyl group (OH) at the end. The 5′ hydroxyl oligos are then triphosphorylated using bis(tri-n-butylammonium) dihydrogen pyrophosphate. A scavenger such as morpholine can be included in the phosphorylation reaction for 5′-triphosphate DNA to prevent byproduct formation, although this does not work for 5′-triphosphate RNA. Finally, the oligos are cleaved and deprotected with ammonia, a step that must be performed at room temperature to minimize the formation of 5′-monophosphate or 5′-diphosphate byproducts [1]. The synthesized oligos are subsequently purified using HPLC and verified with mass spectrometry for quality control.
What are the common impurities associated with this modification and can Synoligo reduce impurities for their synthesis process?
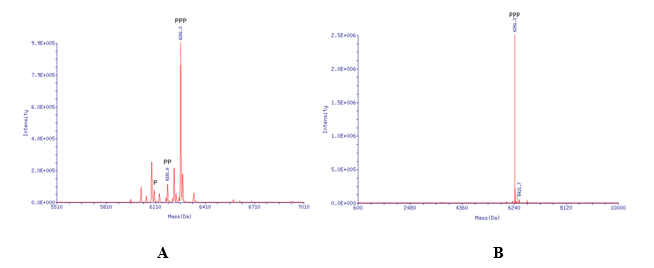
Impurities such as 5′-monophosphate and 5′-diphosphate are commonly formed during synthesis, particularly when using the Ludwig and Eckstein method. Image 2 shows the formation of mono- and diphosphate in a crude mixture before process optimization. With our newly developed in-house synthesis method, Synoligo can deliver more than 90% pure 5′ triphosphate oligonucleotides with any length and modification patterns up to a few hundred of grams scale, free from monophosphate or diphosphate contaminants.
References:
- Grant. Chemical Triphosphorylation of Oligonucleotides. Jove.com. Published June 2, 2022. https://app.jove.com/t/63877
- Sarac I, Meier C. Efficient Automated Solid‐Phase Synthesis of DNA and RNA 5′‐Triphosphates. Chemistry. 2015;21(46):16421-16426. doi: https://doi.org/10.1002/chem.201502844
